6.02 X 10^23
Provided to YouTube by DistroKid602 x 1023 Bop LouieRISING SUN VOLUME ONE GoobGeeb RecordsReleased on. Thus its value is much closer to 6022 1023 than to 6023 1023.
The Number Of N Atoms Is 681 G Of C7h5n3o6 Is X X 10 21 The Value Of X Is N A 6 02 X 10 23 Mol 1 Sarthaks Econnect Largest Online Education Community
10²³ 100000000000000000000000 23 zeroes 60223602100000000000000000000000.
(8.65x10^{4})&line2=\mathrm{Solution:\:}5.2073E28x^{2})
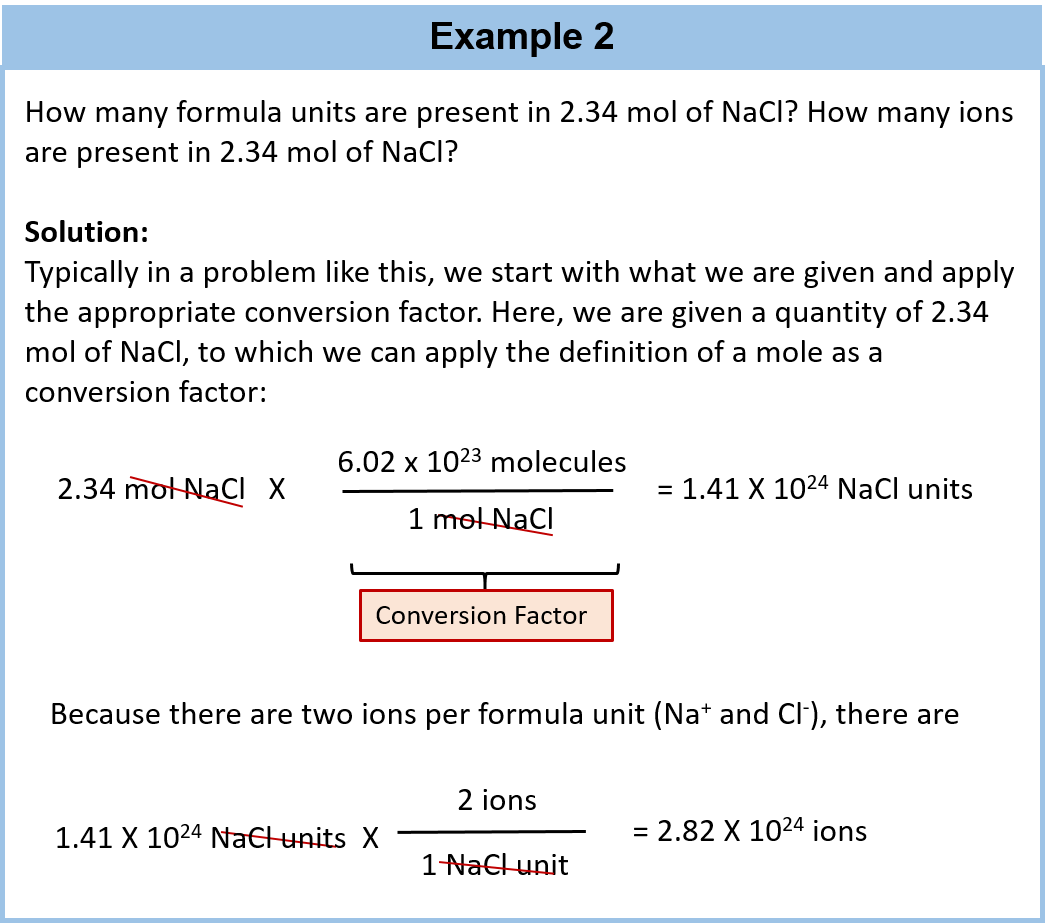
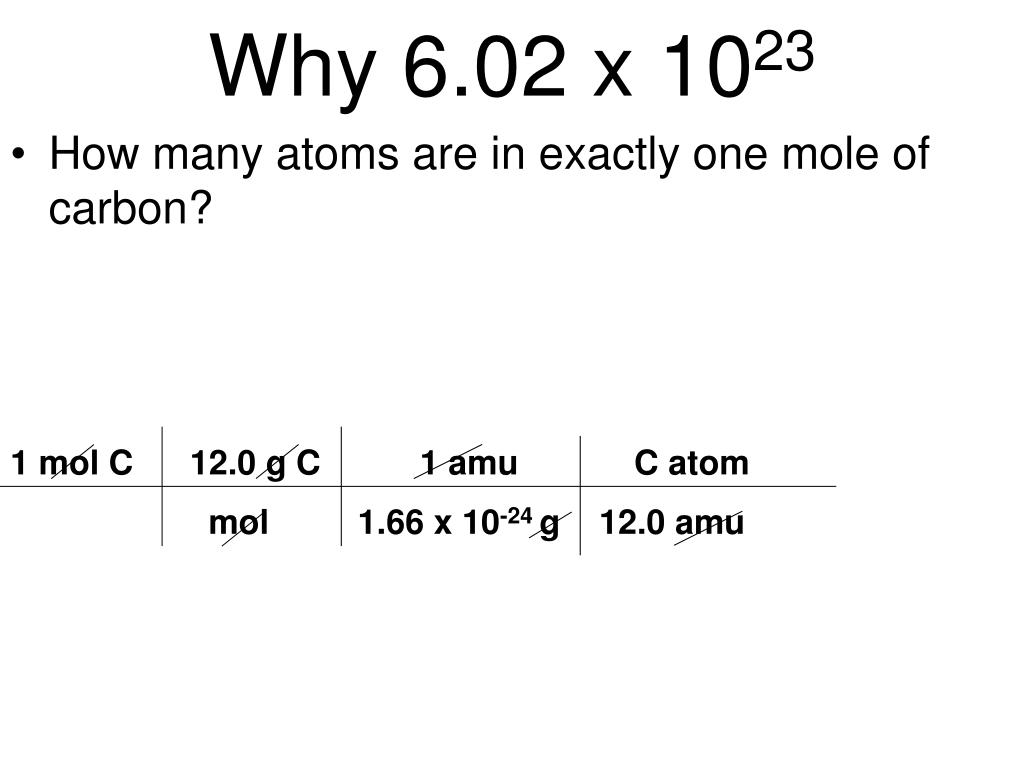
. It is the number of atoms ions and molecules in one gram atom of element one gram molecules of compound and one gram ions of a substance. 1g 1166 10²⁴ amu 1g 602 10²³ amu suppose now we want to check number atoms of carbon in 12 grams of carbon because 12 grams is its molar mass 12g 602 10²³ 12 amu. The SI value of a chemical entity such as atoms electrons or protons is known as the mole abbreviated mol.
To the nearest orderof magnitude how many moles of atoms are in a large domesticcat. The number of atoms in a 12g sample of C-12 B. A mole of atoms is 602 x 1023 atoms.
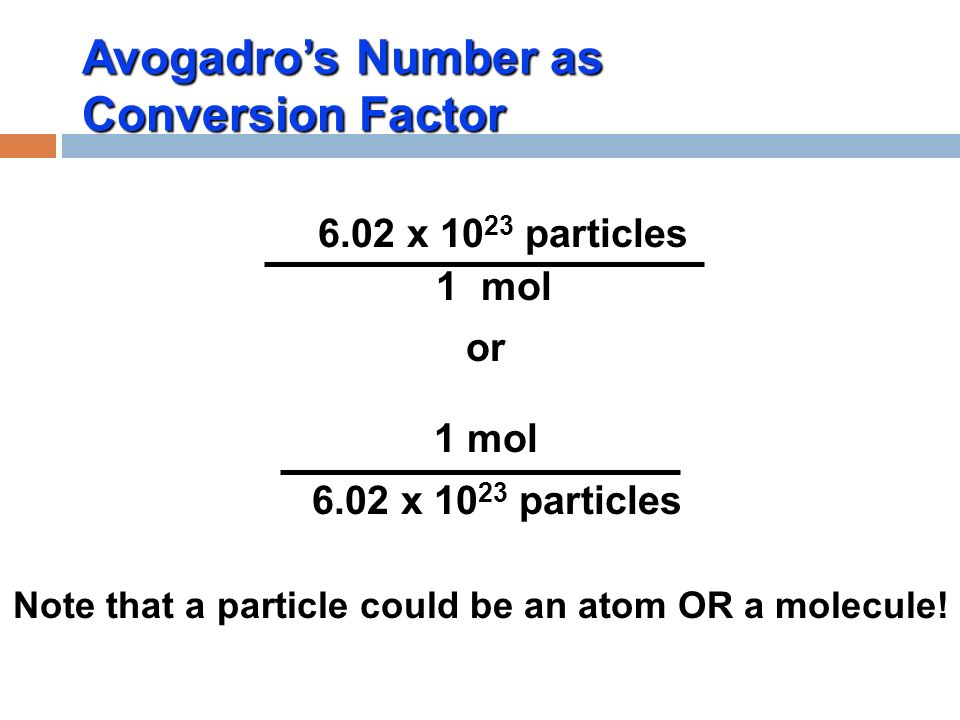
If you have 602 x 10 23 molecules of water in a glass how many moles do you have. Avogadro number connects amu and grams. A mole of a substance can be defined as.
So they started by defining. The number 6022 10²³ is. 10110 1 zero 102100 2 zeroes etc 1023.
The amount of substance that contains 6021023. How to use this calculator. 602 Times 10 23 6.
It refers to the amount of particles in a. According to the latest measurements it has a value of 6022140781023 0000000181023 mol-1. Why is the number 6022 x 10 23.
Basically dealing with the weights of atoms and molecules individually is next to impossible. Therefore 602 x 10²³ seconds 602 x 10²³ seconds 315360000 seconds 1909 x 10⁸ x 10²³ 1909 x 10¹⁵ decades. 480 grams sulfur 1 mole S3207 grams 6022 X 10231 mole S 901 X 1023 atoms of sulfur How many atoms are 602 grams of sulfur.
One mole of one substance contains 602 x 10 23 textbf23 23 atoms or molecules. Step 2 2 of 3. 1225 10 5 3655 10 3 126155 x 10 5.
Convert to Regular Notation 6021023 602 1023 602 10 23 Since the exponent of the scientific notation is positive move the decimal point 23 23 places to the right. What is 6023 1023. This number is called the Avogadro number.
602 grams sulfur 1mole S3207. 1 2 3 4 Chemistry 1 Answer Kai Feb 28 2018 You have one mole of H 2O molecules. The masses of a hydrogen atom.

The Mole Concept Avogadros Constant Mr Carson S Science Page
Chapter 6 Quantities In Chemical Reactions Chemistry

Mole Problems Call Avogadro 6 02 X 10 3 Periodic Table Of Elements Undated Planner Weekly Monthly No Year Pocket Calendar Medium 6x9 Softcover Walmart Com
Calculator Help Griger Science
Ppt Chapter 8 The Mole Part 1 Powerpoint Presentation Free Download Id 5435580
Star Moles Episode 6 02 X 10 23 Schooltube Safe Video Sharing And Management For K12
How Do You Convert 5 3 10 25 Molecules Of Co 2 To Moles Socratic

6 02 X 10 23 Stickers Cafepress
Avogadro S Number 6 02 X 10 23 National Mole Day
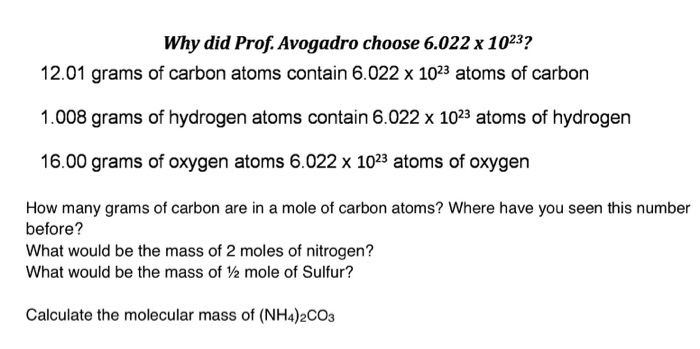
Solved Why Did Prof Avogadro Choose 6 022 X 1023 12 01 Chegg Com
Happy Mole Day 10 22 2020 Technical Staffing Quality Consulting Laboratory Information Management Systems And Training News For Labtopia In Houston Texas Usa

Solved A Mole Of Atoms Is 6 02 X 1023 Atoms How Many Moles Of Atoms Are In A Domestic Cat Wit Amass Of 10 Kg Take The Mass Of An Average Atom

Mole Concept Powerpoint Slides

How Many Grams Of Ta Are There In 6 022 X 10 24 Atoms Of Tantalum Socratic
Massachusetts Institute Of Technology Mit On Twitter October 23rd Is The Day We Celebrate Avogadro S Constant 6 02 X 10 23 Happy Moleday Http T Co Hdfpi0yaqx Twitter
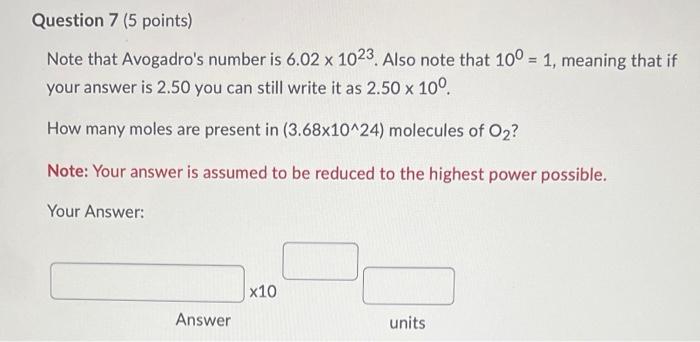
Solved Note That Avogadro S Number Is 6 02 Times 10 23 Chegg Com
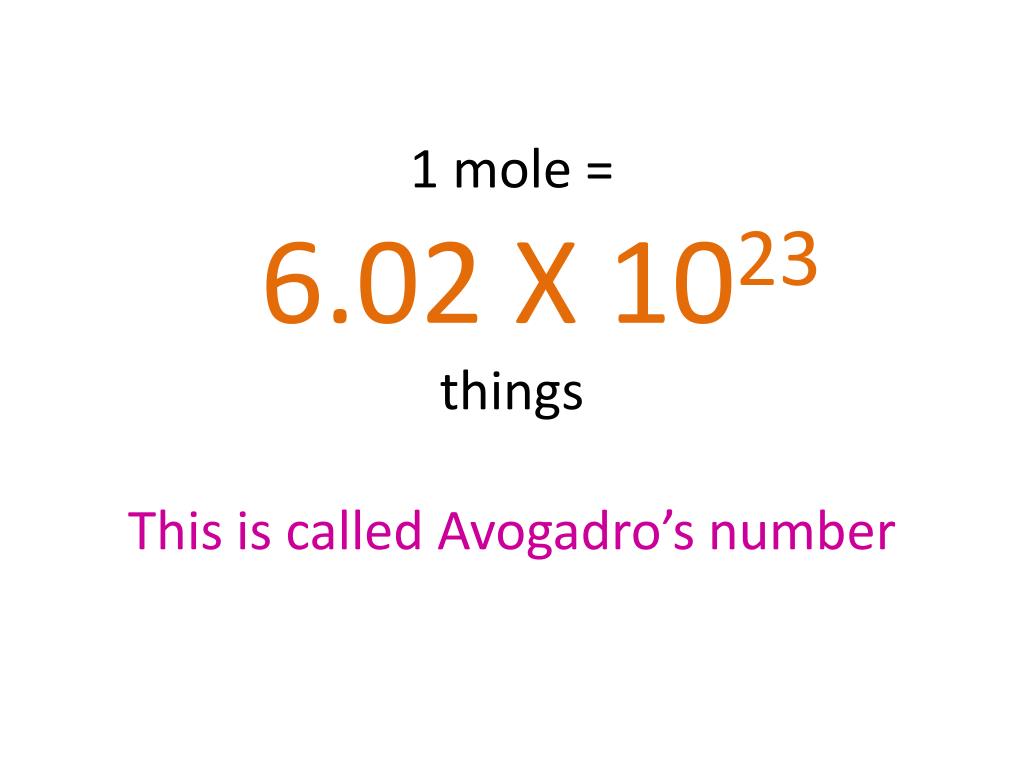
Ppt 1 Mole 6 02 X 10 23 Things This Is Called Avogadro S Number Powerpoint Presentation Id 4272623